NCERT Solutions for Class 12 Chemistry Chapter 14 Biomolecules
NCERT Solutions for Class 12 Chemistry Chapter 14 Biomolecules
Chapter: Chapter 14 – Biomolecules
Question 14.1:
Glucose or sucrose are soluble in water but cyclohexane or benzene (simple six membered ring compounds) are insoluble in water. Explain.
Answer
A glucose molecule contains five −OH groups while a sucrose molecule contains eight
−OH groups. Thus, glucose and sucrose undergo extensive H-bonding with water. Hence, these are soluble in water. But cyclohexane and benzene do not contain −OH groups. Hence, they cannot undergo H-bonding with water and as a result, are insoluble in water.
Question 14.2:
What are the expected products of hydrolysis of lactose?
Answer

Question 14.5:
Where does the water present in the egg go after boiling the egg?
Answer
When an egg is boiled, the proteins present inside the egg get denatured and coagulate.
After boiling the egg, the water present in it is absorbed by the coagulated protein through H-bonding.
Question 14.6:
Why cannot vitamin C be stored in our body?
Answer
Vitamin C cannot be stored in our body because it is water soluble. As a result, it is readily excreted in the urine.
Question 14.7:
What products would be formed when a nucleotide from DNA containing thymine is hydrolysed?
Answer
When a nucleotide from the DNA containing thymine is hydrolyzed, thymine β-D-2- deoxyribose and phosphoric acid are obtained as products.
Question 14.8:
When RNA is hydrolysed, there is no relationship among the quantities of different bases obtained. What does this fact suggest about the structure of RNA?
Answer
A DNA molecule is double-stranded in which the pairing of bases occurs. Adenine always pairs wiymine, while cytosine always pairs with guanine. Therefore, on hydrolysis of DNA, the quantity of adenine produced is equal to that of thymine and similarly, the quantity of cytosine is equal to that of guanine. But when RNA is hydrolyzed, there is no relationship among the quantities of the different bases obtained. Hence, RNA is single-stranded.
Question 14.1:
What are monosaccharides?
Answer
Monosaccharides are carbohydrates that cannot be hydrolysed further to give simpler units of polyhydroxy aldehyde or ketone. Monosaccharides are classified on the bases of number of carbon atoms and the functional group present in them. Monosaccharides containing an aldehyde group are known as aldoses and those containing a keto group are known as ketoses. Monosaccharides are further classified as trioses, tetroses, pentoses, hexoses, and heptoses according to the number of carbon atoms they contain. For example, a ketose containing 3 carbon atoms is called ketotriose and an aldose containing 3 carbon atoms is called aldotriose.
Question 14.2:
What are reducing sugars?
Answer
Reducing sugars are carbohydrates that reduce Fehling’s solution and Tollen’s reagent. All monosaccharides and disaccharides, excluding sucrose, are reducing sugars.
Question 14.3:
Write two main functions of carbohydrates in plants.
Answer
Two main functions of carbohydrates in plants are:
(i) Polysaccharides such as starch serve as storage molecules.
(ii) Cellulose, a polysaccharide, is used to build the cell wall.
Question 14.4:
Classify the following into monosaccharides and disaccharides.
Ribose, 2-deoxyribose, maltose, galactose, fructose and lactose
Answer
Monosaccharides: Ribose, 2-deoxyribose, galactose, fructose
Disaccharides: Maltose, lactose
Question 14.5:
What do you understand by the term glycosidic linkage?
Answer
Glycosidic linkage refers to the linkage formed between two monosaccharide units through an oxygen atom by the loss of a water molecule.
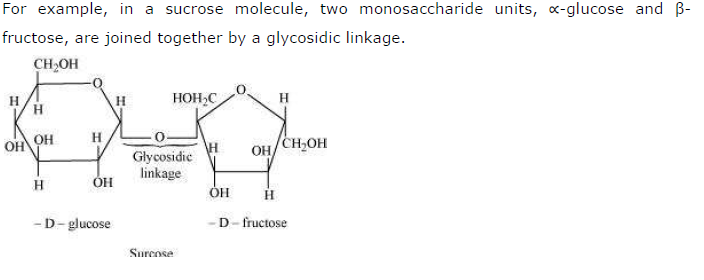
Question 14.6:
What is glycogen? How is it different from starch?
Answer
Glycogen is a carbohydrate (polysaccharide). In animals, carbohydrates are stored as glycogen.
Starch is a carbohydrate consisting of two components − amylose (15 − 20%) and amylopectin (80 − 85%).
However, glycogen consists of only one component whose structure is similar to amylopectin. Also, glycogen is more branched than amylopectin.
Question 14.7:
What are the hydrolysis products of (i) sucrose and (ii) lactose?
Answer

Question 14.10:
Enumerate the reactions of D-glucose which cannot be explained by its open chain structure.
Answer
(1) Aldehydes give 2, 4-DNP test, Schiff’s test, and react with NaHSO4 to form the hydrogen sulphite addition product. However, glucose does not undergo these reactions.
(2) The pentaacetate of glucose does not react with hydroxylamine. This indicates that a free −CHO group is absent from glucose.
(3) Glucose exists in two crystalline forms − ∝ andβ. The ∝-form (m.p. = 419 K) crystallises from a concentrated solution of glucose at 303 K and the β-form (m.p = 423
K) crystallises from a hot and saturated aqueous solution at 371 K. This behaviour cannot be explained by the open chain structure of glucose.
Question 14.11:
What are essential and non-essential amino acids? Give two examples of each type.
Answer
Essential amino acids are required by the human body, but they cannot be synthesised in the body. They must be taken through food. For example: valine and leucine
Non-essential amino acids are also required by the human body, but they can be synthesised in the body. For example: glycine, and alanine
Question 14.12:
Define the following as related to proteins
(i) Peptide linkage (ii) Primary structure (iii) Denaturation.
Answer
(i) Peptide linkage:
The amide formed between −COOH group of one molecule of an amino acid and −NH2 group of another molecule of the amino acid by the elimination of a water molecule is called a peptide linkage.
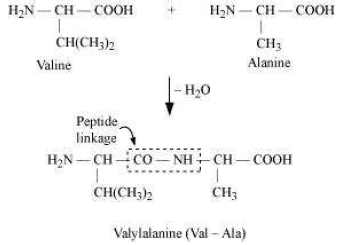
(ii) Primary structure:
The primary structure of protein refers to the specific sequence in which various amino acids are present in it, i.e., the sequence of linkages between amino acids in a polypeptide chain. The sequence in which amino acids are arranged is different in each protein. A change in the sequence creates a different protein.
(iii) Denaturation:
In a biological system, a protein is found to have a unique 3-dimensional structure and a unique biological activity. In such a situation, the protein is called native protein.
However, when the native protein is subjected to physical changes such as change in temperature or chemical changes such as change in pH, its H-bonds are disturbed. This disturbance unfolds the globules and uncoils the helix. As a result, the protein loses its biological activity. This loss of biological activity by the protein is called denaturation.
During denaturation, the secondary and the tertiary structures of the protein get destroyed, but the primary structure remains unaltered. One of the examples of denaturation of proteins is the coagulation of egg white when an egg is boiled.
Question 14.13:
What are the common types of secondary structure of proteins?
Answer
There are two common types of secondary structure of proteins:
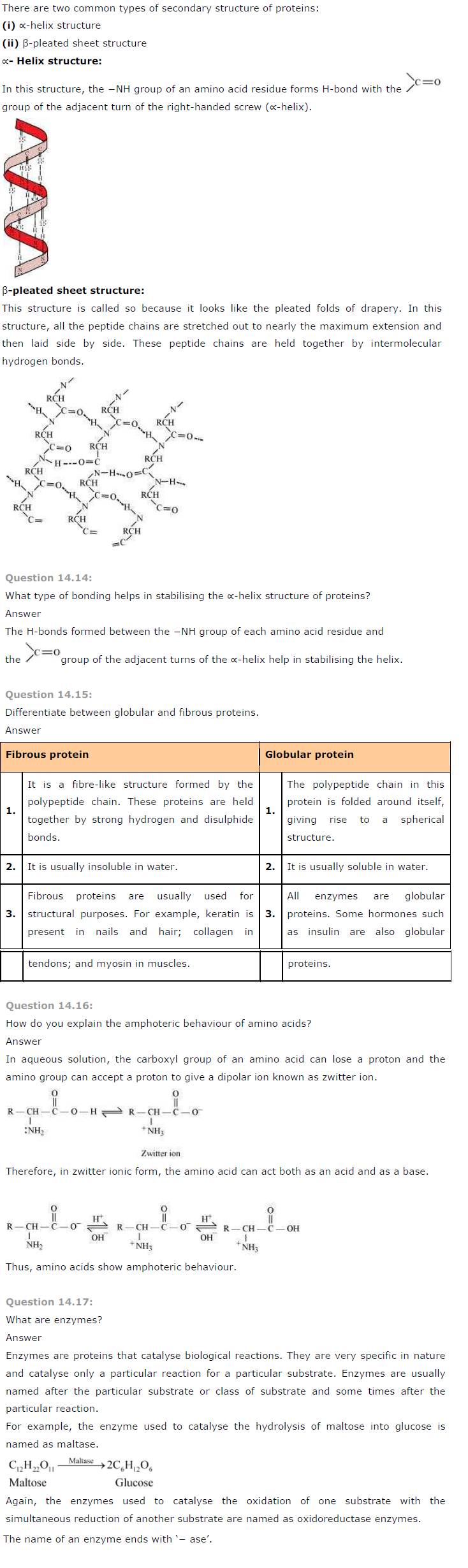
Question 14.18:
What is the effect of denaturation on the structure of proteins?
Answer
As a result of denaturation, globules get unfolded and helixes get uncoiled. Secondary and tertiary structures of protein are destroyed, but the primary structures remain unaltered. It can be said that during denaturation, secondary and tertiary-structured proteins get converted into primary-structured proteins. Also, as the secondary and tertiary structures of a protein are destroyed, the enzyme loses its activity.
Question 14.19:
How are vitamins classified? Name the vitamin responsible for the coagulation of blood.
Answer
On the basis of their solubility in water or fat, vitamins are classified into two groups.
(i) Fat-soluble vitamins: Vitamins that are soluble in fat and oils, but not in water, belong to this group. For example: Vitamins A, D, E, and K
(ii) Water-soluble vitamins: Vitamins that are soluble in water belong to this group.
For example: B group vitamins (B1, B2, B6, B12, etc.) and vitamin C
However, biotin or vitamin H is neither soluble in water nor in fat.
Vitamin K is responsible for the coagulation of blood.
Question 14.20:
Why are vitamin A and vitamin C essential to us? Give their important sources.
Answer
The deficiency of vitamin A leads to xerophthalmia (hardening of the cornea of the eye) and night blindness. The deficiency of vitamin C leads to scurvy (bleeding gums).
The sources of vitamin A are fish liver oil, carrots, butter, and milk. The sources of vitamin C are citrus fruits, amla, and green leafy vegetables.
Question 14.21:
What are nucleic acids? Mention their two important functions.
Answer
Nucleic acids are biomolecules found in the nuclei of all living cells, as one of the constituents of chromosomes. There are mainly two types of nucleic acids − deoxyribonucleic acid (DNA) and ribonucleic acid (RNA). Nucleic acids are also known as polynucleotides as they are long-chain polymers of nucleotides.
Two main functions of nucleic acids are:
(i) DNA is responsible for the transmission of inherent characters from one generation to the next. This process of transmission is called heredity.
(ii) Nucleic acids (both DNA and RNA) are responsible for protein synthesis in a cell.
Even though the proteins are actually synthesised by the various RNA molecules in a cell, the message for the synthesis of a particular protein is present in DNA.
Question 14.22:
What is the difference between a nucleoside and a nucleotide?
Answer
A nucleoside is formed by the attachment of a base to 1′ position of sugar.
Nucleoside = Sugar + Base
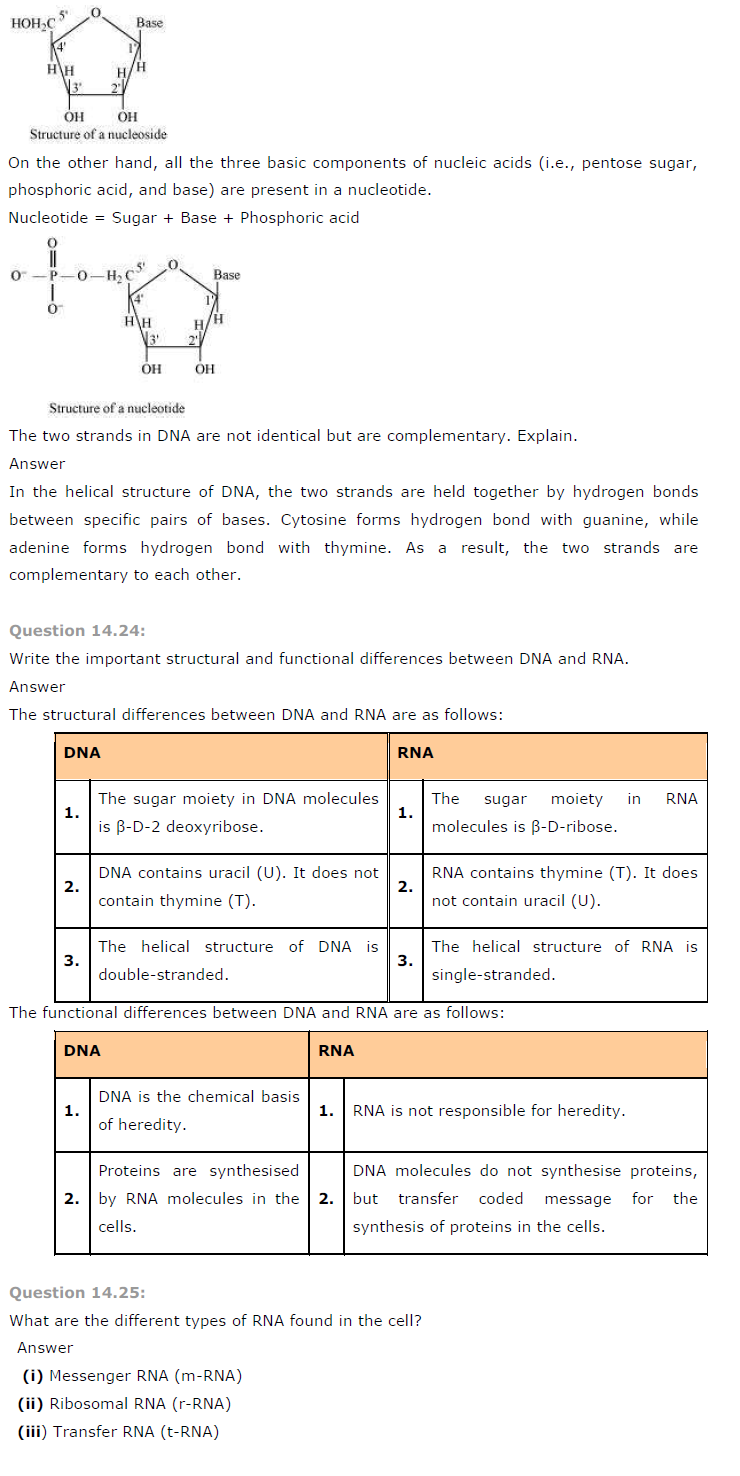
NCERT Solutions For Class 12 Chemistry PDF Download
- Chapter 1 The Solid State NCERT Solutions Class 12 Chemistry
- Chapter 2 Solutions NCERT Solutions Class 12 Chem
- Ch 3 Electro chemistry NCERT Solutions Class 12 Chemistry
- Chapter 4 Chemical Kinetics NCERT Solutions Class 12 Chem
- Chapter 5 Surface Chemistry Class 12 Chemistry Download
- Ch 6 General Principles and Processes of Isolation of Elements Chemistry Download
- Chapter 7 The p Block Elements NCERT Solutions Class 12 Chemistry
- Chapter 8 The d and f Block Elements NCERT Solutions Class 12th
- Ch 9 Coordination Compounds NCERT Solutions Class 12
- Chapter 10 Haloalkanes and Haloarenes NCERT Chemistry
- Chapter 11 Alcohols Phenols and Ethers NCERT Chemistry Download
- Ch 12 Aldehydes Ketones and Carboxylic Acids NCERT Solutions Class 12
- Chapter 13 Amines NCERT Solutions Class 12 Chemistry
- Chapter 14 Biomolecules NCERT Solutions Class 12 Chemistry
- Ch 15 Polymers NCERT Solutions Chemistry Download
- Chapter 16 Chemistry in Everyday Life Chemistry Download
NCERT Solutions For Class 12 PDF Download
NCERT Solutions For Class 12 for subjects Maths , Physics , Biology , Chemistry , Accountancy , Business Studies , Economics , English Flamingo , English Vista , Psychology are available for download. The solutions available are of the ncert books for class 12th.
- Class 12 Maths Solutions
- NCERT Solutions For Class 12 Physics Solutions
- NCERT Solutions For Class 12 Biology Solutions
- Class 12 Chemistry Solutions
- NCERT Solutions For Class 12 Accountancy Solutions
- NCERT Solutions For Class 12 Business Studies Solutions
- Class 12 Economics Solutions
- NCERT Solutions For Class 12 English Flamingo Solutions
- NCERT Solutions For Class 12 English Vista Solutions
- Class 12 Psychology Solutions
NCERT Books Class 12 PDF Download
NCERT Books For Class 12th for Accountancy, Biology, Business Studies, Chemistry , Economics , English , Geography , Hindi , History , Mathematics , Physics , Political Science , Psychology , Sanskrit , Sociology , Urdu in PDF are available for download here .
- Accountancy NCERT Books For Class 12th
- Biology NCERT Books For Class 12th
- Business Studies NCERT Books For Class 12th
- Chemistry NCERT BOOKS For Class 12
- Economics NCERT BOOKS For Class 12
- English NCERT BOOKS For Class 12
- Geography NCERT BOOKS For Class 12
- Hindi NCERT BOOKS For Class 12
- History NCERT BOOKS For Class 12
- Mathematics NCERT BOOKS For Class 12
- Physics NCERT BOOKS For Class 12
- Political Science NCERT BOOKS For Class 12
- Psychology NCERT BOOKS For Class 12
- Sanskrit NCERT BOOKS For Class 12
- Sociology NCERT BOOKS For Class 12
- Urdu NCERT BOOKS For Class 12
NCERT Solutions for Class 12 Chemistry Chapter 7 The p Block Elements
Discuss the general characteristics of Group 15 elements with reference ...
NCERT Solutions for Class 12 Chemistry Chapter 3 Electrochemistry
Chapter: Chapter 3 – Electrochemistry Question 3.1:
Arrange the following metals in the order in which they displace each other from the solution of their salts.
Al, Cu, Fe, Mg and Zn
...
NCERT Solutions for Class 12 Chemistry Chapter 2 Solutions
Chapter: Chapter 2 – Solutions Question 2.23:
Suggest the most important type of intermolecular attractive interaction in the following pairs.
(i) n-hexane and n-octane
(ii) I2 and CCl4
(iii) NaClO4 and water
...
NCERT Solutions for Class 12 Chemistry Chapter 9 Coordination Compounds
Chapter: Chapter 9 – Coordination Compounds Question 9.1:
Explain the bonding in coordination compounds in terms of Werner’s postulates.
Answer
Werner’s postulates explain the bonding in coordination compounds as follows:
...
NCERT Solutions for Class 12 Chemistry Chapter 16 Chemistry in Everyday Life
Chapter:Chapter 16 – Chemistry in Everyday Life Question 16.1: Why do we need to classify drugs in different ways?
Answer
The classification of drugs and the ...
NCERT Solutions for Class 12 Chemistry Chapter 6 General Principles and Processes of Isolation of Elements
Chapter: Chapter 6 – General Principles and Processes of Isolation of Elements Question 6.1:
Copper can be extracted ...